Oral Presentation 28th Annual Lorne Proteomics Symposium 2023
The epididymis: a window for relaying stress signals to the male germline and potential offspring (#2)
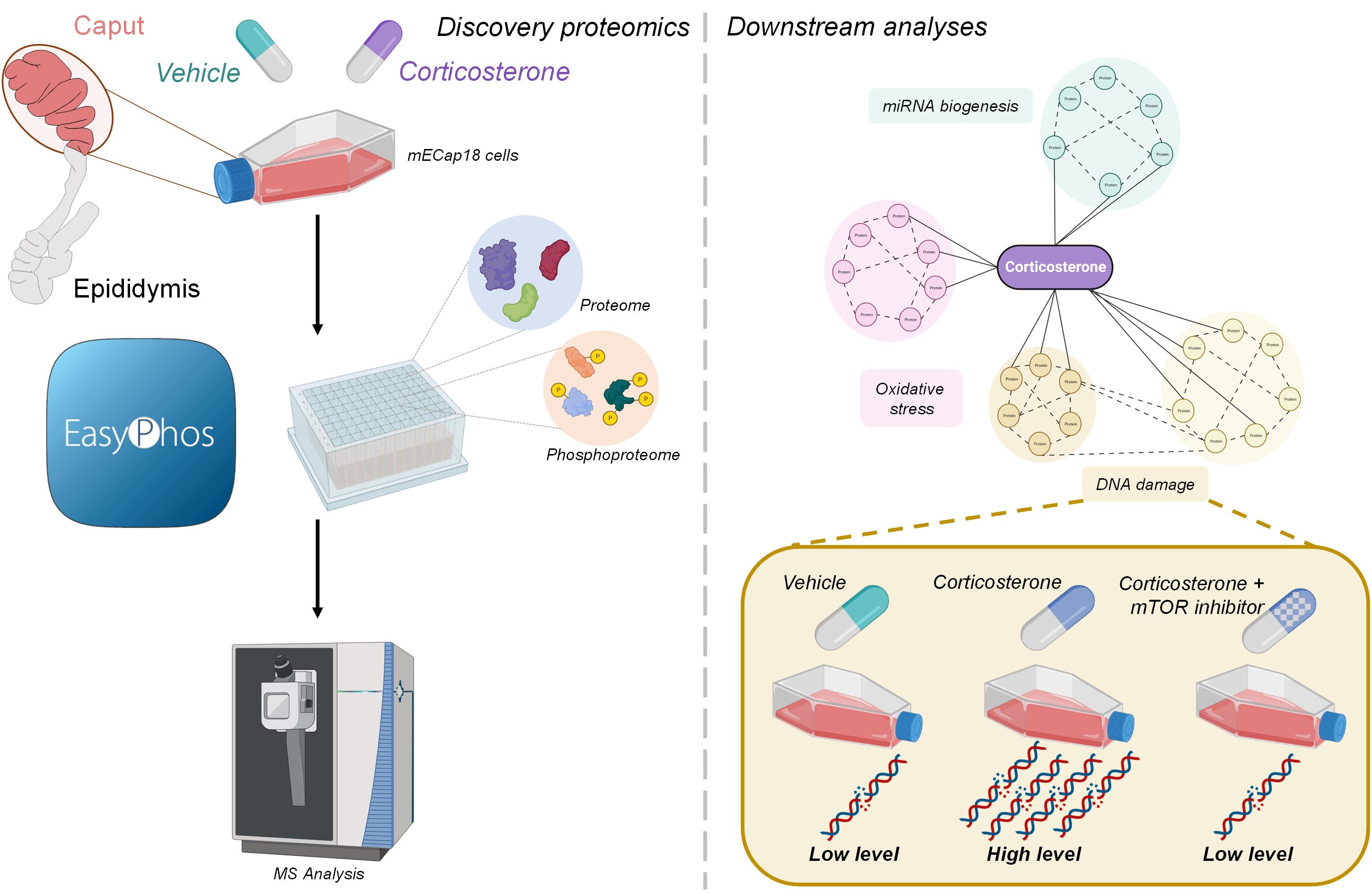
Preconception paternal health has increasingly been linked to a spectrum of offspring health outcomes. Indeed, preclinical models have demonstrated a periconception legacy of paternal trauma that manifests in offspring as impaired stress-relevant behavioural and physiological responses. Our group previously reported that a chronic low-dose corticosterone challenge of male mice produced increased anxiety-relevant behaviours in offspring. Although sperm passage through the male reproductive tract (epididymis) is proposed to be a putative staging point for relaying stress signals to the male germline, the intricacies of the molecular pathways responsible for this form of communication remain to be fully elucidated. To address this important knowledge gap, here we have capitalised on recent advances in phosphoproteomic analyses to investigate the impact of corticosterone supplementation and consequential corticosteroid receptor downstream signalling in a tractable epididymal epithelial tissue culture system (mECap18 cells). In agreement with no overt change in glucocorticoid receptor protein (NR3C1) levels, we detected only subtle adaptation of the global proteomic profile of mECap18 cells to corticosterone challenge (i.e., 73/4171 proteins). By contrast, ~10% of the mECap18 phosphoproteome was substantially altered following corticosterone exposure. In-silico analysis of the dysregulated parent proteins revealed an activation of pathways linked to DNA repair and oxidative stress responses as well as a reciprocal inhibition of those associated with organismal death. Notably, corticosterone also induced the phosphorylation of several proteins linked to the biogenesis of regulatory microRNAs. Accordingly, orthogonal validation strategies confirmed an increase in DNA damage and an altered abundance profile of a subset of microRNAs in corticosterone-treated cells. Further, we demonstrated the DNA damage burden incurred by corticosterone can be ameliorated via prior and selective kinase inhibition. Together, these data confirm that epididymal epithelial cells are reactive to corticosterone challenge and that their response is tightly coupled to the opposing action of cellular kinases and phosphatases.