Poster Presentation 28th Annual Lorne Proteomics Symposium 2023
The characterisation of protein binding from in vivo paediatric ECMO circuits (#108)
Background:
Extracorporeal Membrane Oxygenation (ECMO) is an artificial heart-lung machine for critically ill patients with severe respiratory or cardiac failure (1). Despite providing critical support, there is an increased risk of bleeding and clotting due to the continuous contact between blood and circuit (2). Whilst previous studies have extensively investigated blood samples from patients on ECMO (3, 4), protein and cellular adsorption to ECMO circuit, as a factor that could potentially contribute to clinical complications, has largely been overlooked.
Aims:
To characterise the proteins bound to ECMO circuits collected from children on ECMO.
Methods:
This study was approved by the Royal Children's Hospital ethics committee (HREC/15/RCHM/123). ECMO circuits were collected when removed from patients. For each circuit, samples from 5 different sites were collected (Figure 1). Protein quantification and characterisation was performed using bicinchoninic acid protein assay and Data-independent acquisition Mass Spectrometry (DIA-MS), respectively. Identified proteins were processed using the Reactome Over-representation Pathway Analyses tool (5) to determine corresponding functional pathways.
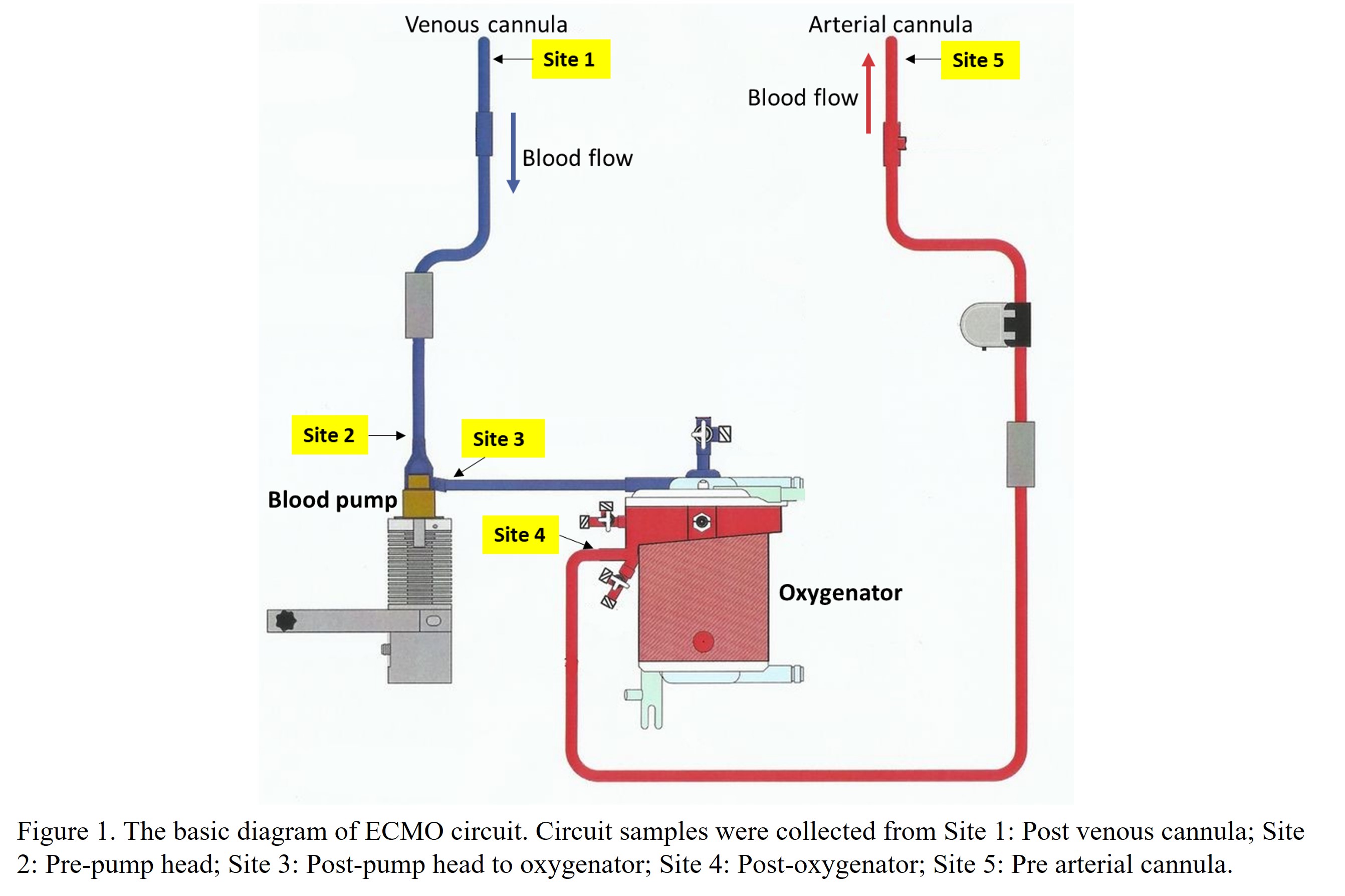
Results:
Seven ECMO circuits were collected from six paediatric patients, with Patient 1 requiring a second run of ECMO support (Named as Patient 1.1 and 1.2).
The median protein concentration of all samples (n=35) was 16.3ug/mL (Range: 0~76.9ug/mL). In site-matched patient comparisons, the protein concentrations of Patient 4 were significantly higher than that of Patient 5, while in patient-matched site comparisons, site 1 samples were significantly higher than site 2, 3 and 5 samples.
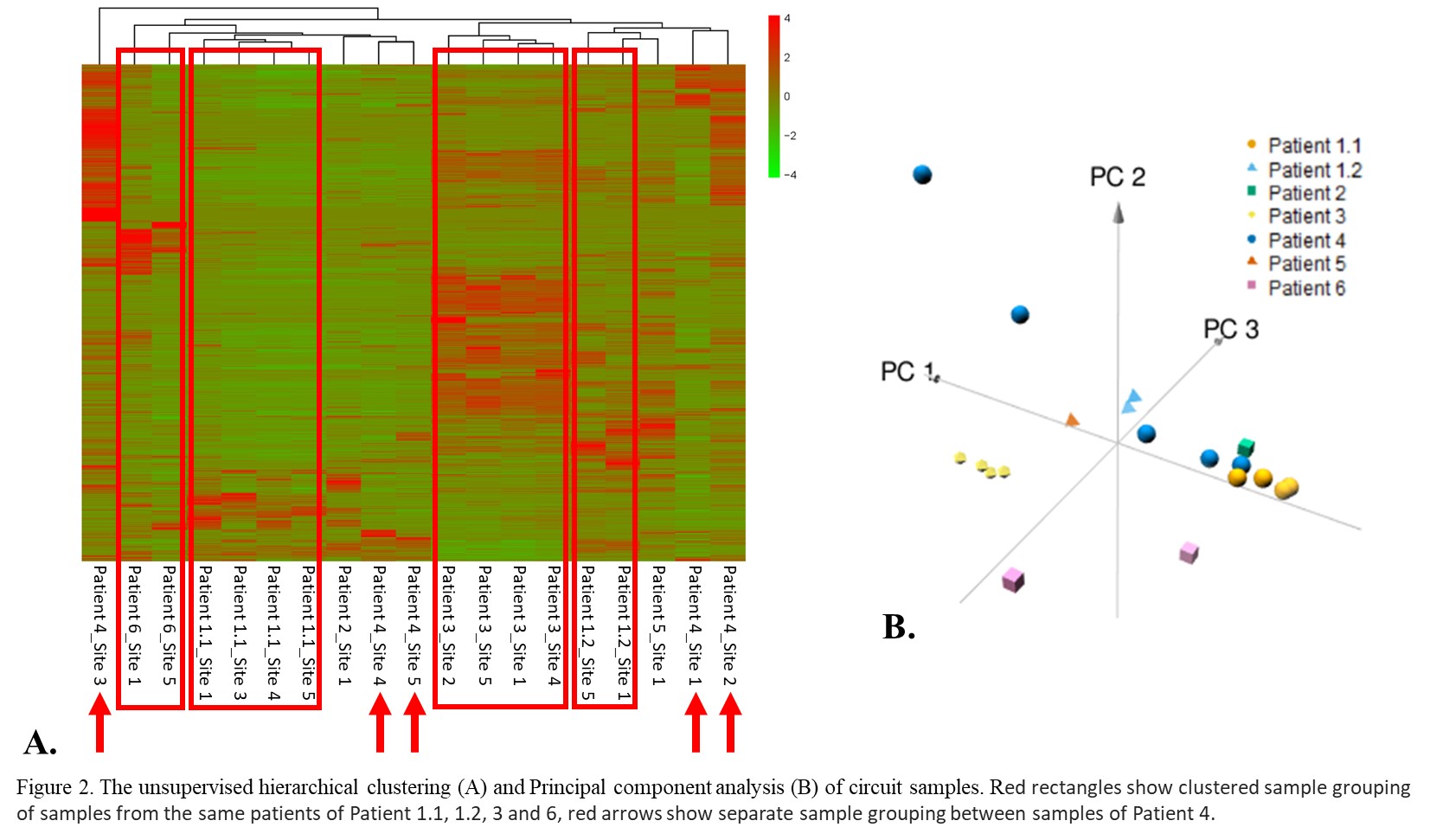
Samples with at least 20ug protein (n=19) were analysed by DIA-MS. The number of proteins identified within each sample varied, with a median of 2138 (Range: 913~3073). Specifically, albumin, haemoglobin, apolipoprotein E, apolipoprotein A-I, fibrinogen, and band 3 anion transport protein were the most abundant proteins identified in most samples. Hierarchical clustering and principal component analysis showed samples from the same ECMO circuit clustered together (Patient 1.1, 1.2, 3 and 6), except in Patient 4 where samples showed significant differences between sites (Figure 2). The pathway analysis demonstrated that identified proteins were enriched in pathways related to cell cycle, protein metabolism, coagulation, and inflammation.
Conclusions:
This is the first study to characterise the proteins bound to ECMO circuits collected from paediatric patients utilising proteomic approaches. Composition of bound proteins was heterogeneous between different patients and between different sites. The bound proteins were enriched in pathways of coagulation and inflammation, which may be associated with clinical outcomes and provide a potential area for further interventions.
- Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G. Extracorporeal life support: the ELSO red book (5th Edition): Extracorporeal Life Support Organization (ELSO); 2017.
- Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation. Am J Respir Crit Care Med. 2017;196(6):762-71.
- Caprarola SD, Ng DK, Carroll MK, Tekes A, Felling RJ, Salorio CF, et al. Pediatric ECMO: unfavorable outcomes are associated with inflammation and endothelial activation. Pediatr Res. 2022;92(2):549-56.
- Van Den Helm S, Yaw HP, Letunica N, Barton R, Weaver A, Newall F, et al. Platelet Phenotype and Function Changes With Increasing Duration of Extracorporeal Membrane Oxygenation. Crit Care Med. 2022;50(8):1236-45.
- Gillespie M, Jassal B, Stephan R, Milacic M, Rothfels K, Senff-Ribeiro A, et al. The reactome pathway knowledgebase 2022. Nucleic Acids Research. 2022;50(D1):D687-D92.